Pharmaceutical companies are involved in one of the most regulated industries across the globe. Even more, each country has its own compliance regulations that your pharmaceutical company must meet to market your products.
Since the year 2000, pharmaceutical companies have been issued over $113 trillion dollars in penalties and fines. Many of them are because of mislabeling, misleading statements, product safety violations, and false claims.
It’s absolutely critical that pharmaceutical companies create strict brand guidelines to properly represent their products. And by implementing a content governance platform, you can find and identify compliance issues before they become fines – and continue to monitor your content over time.
Acrolinx helps pharmaceutical companies avoid compliance issues and reduce risk every day
Here are 6 steps Acrolinx customers follow when creating pharmaceutical marketing content to avoid compliance issues.
Step 1 – Identify terms we can and can’t use for compliance
First we need to understand what terminology we’re able to use in our messaging, which terms we can’t use, which terms are commonly misused, and how context impacts the usage of those terms. Often this is an existing list your compliance team can provide. This includes terms focused on:
- Proper branding and tagline usage per locale
- Absolute claim terminology like “cure”
- Dosages and unit measurements
- Market research terminology
Step 2 – Collect important disclaimers and messaging
Like terminology, you must write disclaimers, instructions, and market messaging exactly as approved by your compliance department. Without these guidelines, messaging can vary across advertisements, web content, print material and more, which can confuse customers and misrepresent patient outcomes. Your compliance team should also be able to provide approved messaging for:
- Product disclaimers
- Alerts and warnings
- Side effect phrasing
- Customer testimonials
Step 3 – Upload important terms and messaging into Acrolinx’s content governance platform
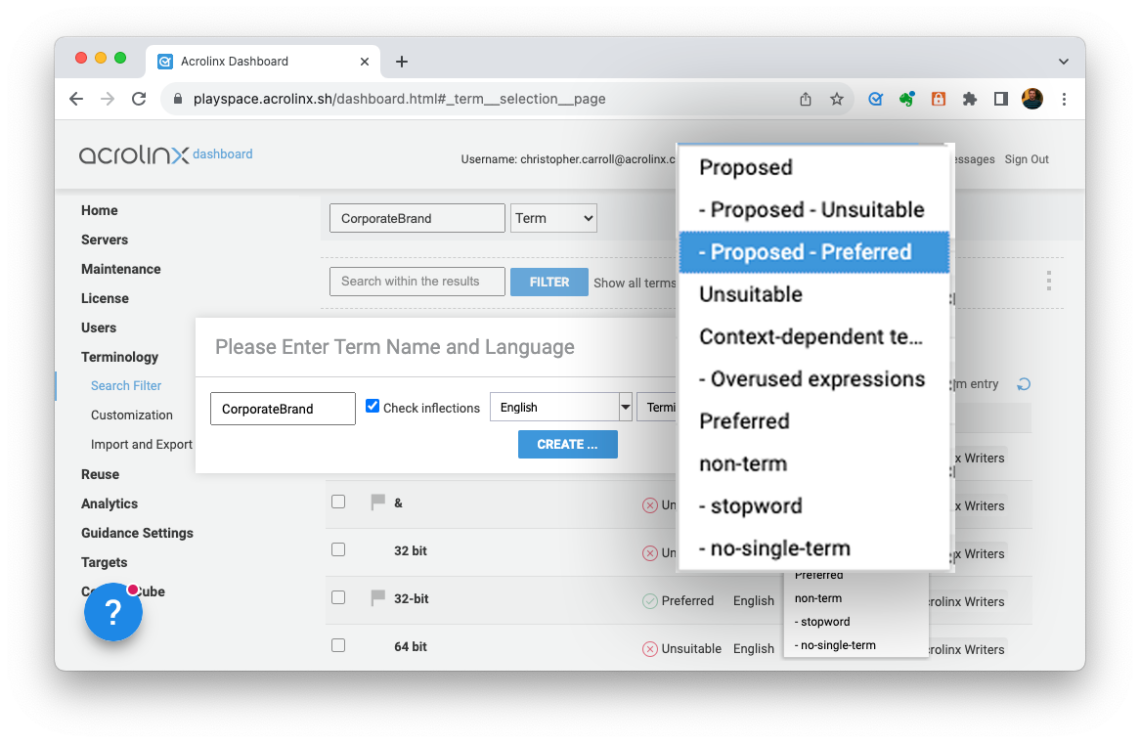
You can add or bulk upload important terminology and messaging into Acrolinx. This includes unapproved terms, phrases, and messaging, which you can then link to preferred variants. This makes sure that your critical warnings, disclaimers, branding, and compliance messaging are used correctly. As writers use incorrect variants of these messages, Acrolinx will find them and give writers clickable replacements to keep content compliant.
Acrolinx uses audience targets to create focused writing guidelines for different languages, locales, use cases, and industries. You can configure each target to find compliance issues and issues with scannability, clarity, inclusivity, consistency, tone of voice, and more.
BONUS:
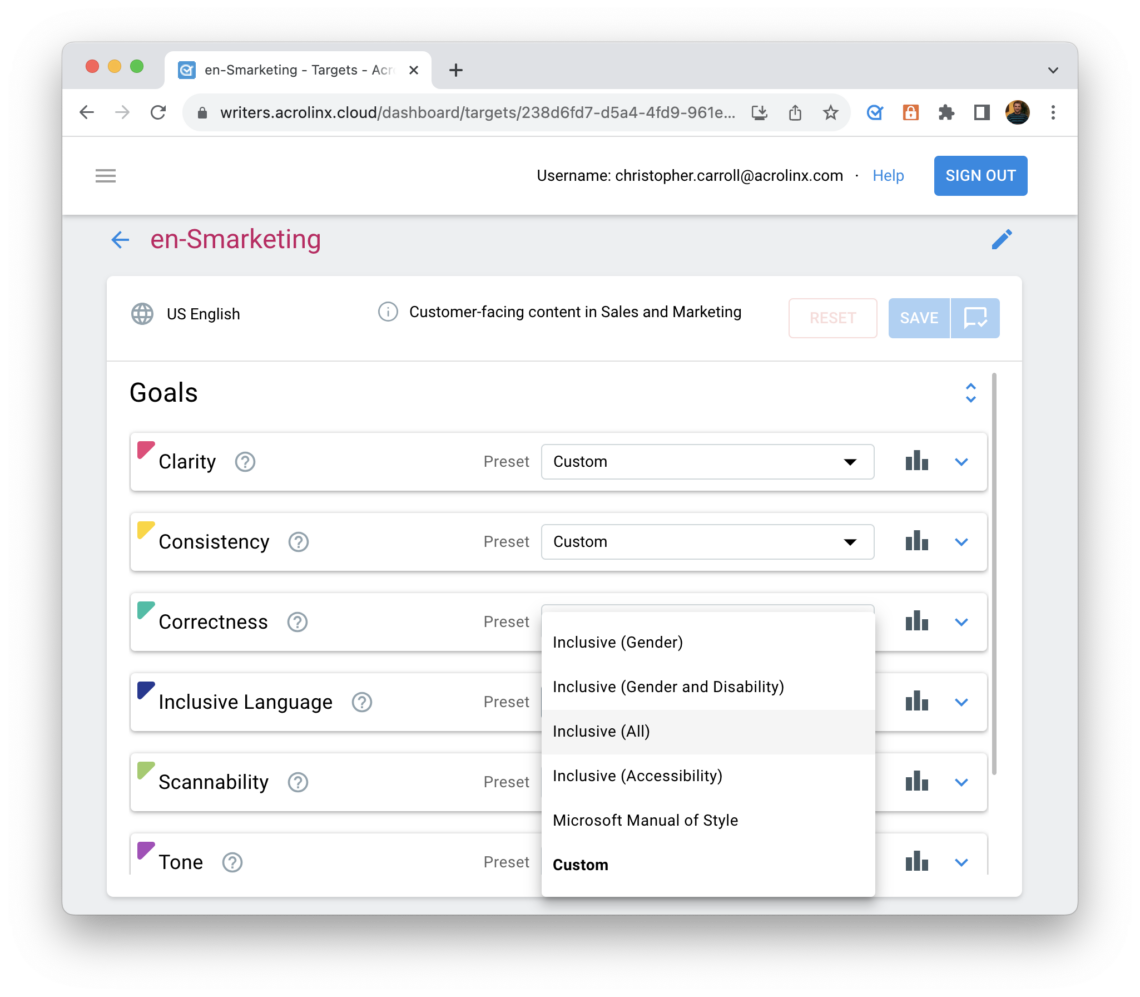
This is a great opportunity to identify the target audiences your products will serve. Demographics to consider are ages, interests, quality of life, and desired outcomes from your products, such as comfort or cosmetics. These demographics will impact the desired clarity, tone, and terminology of your content, which you can configure in your audience target.
Step 4 – Find compliance issues in your published content
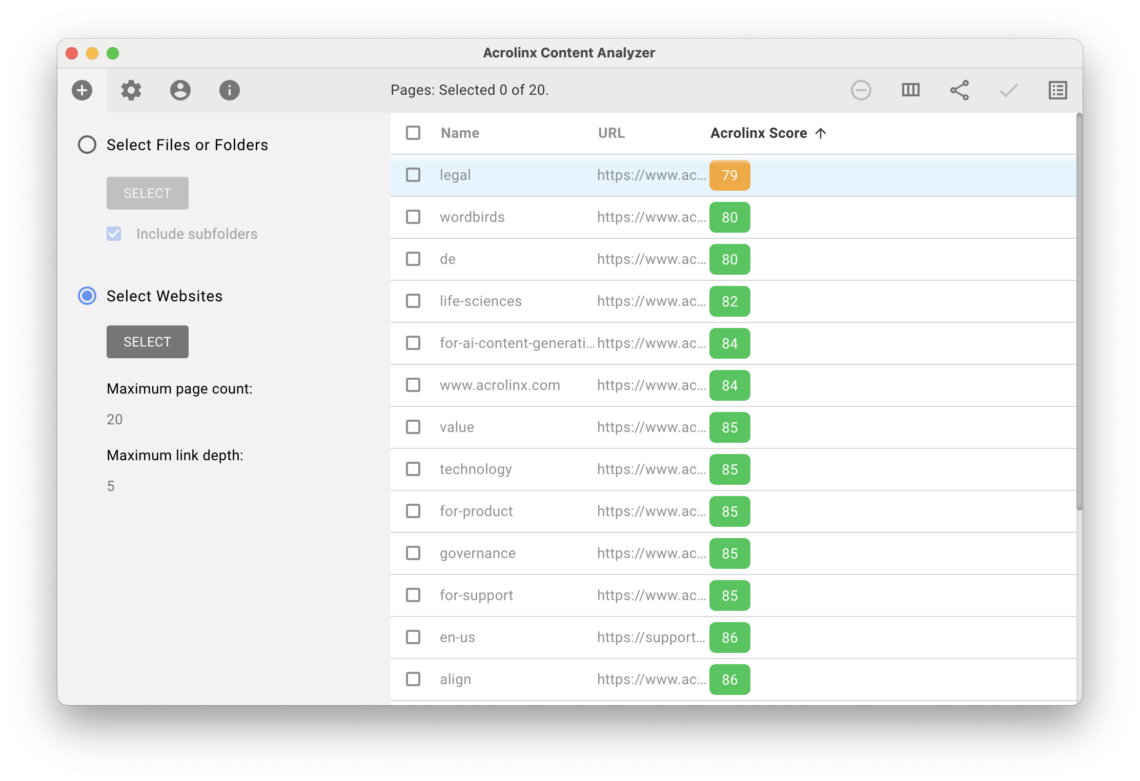
Acrolinx customers have a number of ways to check published content for compliance issues. The Content Analyzer is a great way to spot check a repository of content quickly. The Content Analyzer will provide Acrolinx Scores to help you understand which content pieces have compliance issues. This is a fast and easy way to analyze any gaps you have in your content.
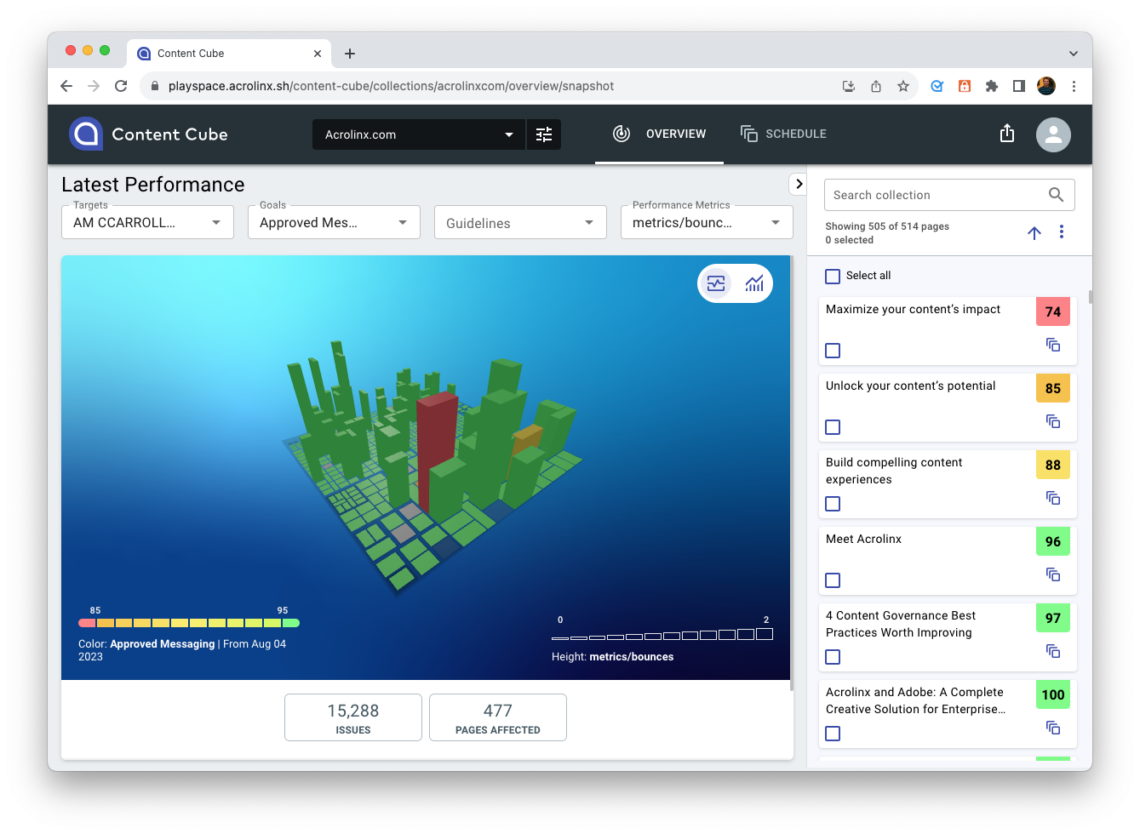
For a more strategic approach, and to support your content governance strategy, Acrolinx can continually check your content every week for compliance and quality issues. Published web pages receive Acrolinx quality scores. You can then prioritize these pages by issue type, such as compliance issues. You can then export these lists and send them to the content team. Each export includes detailed information on the type of content, where it’s located, and the exact issue to correct.
Step 5 – Fix compliance issues before you publish them
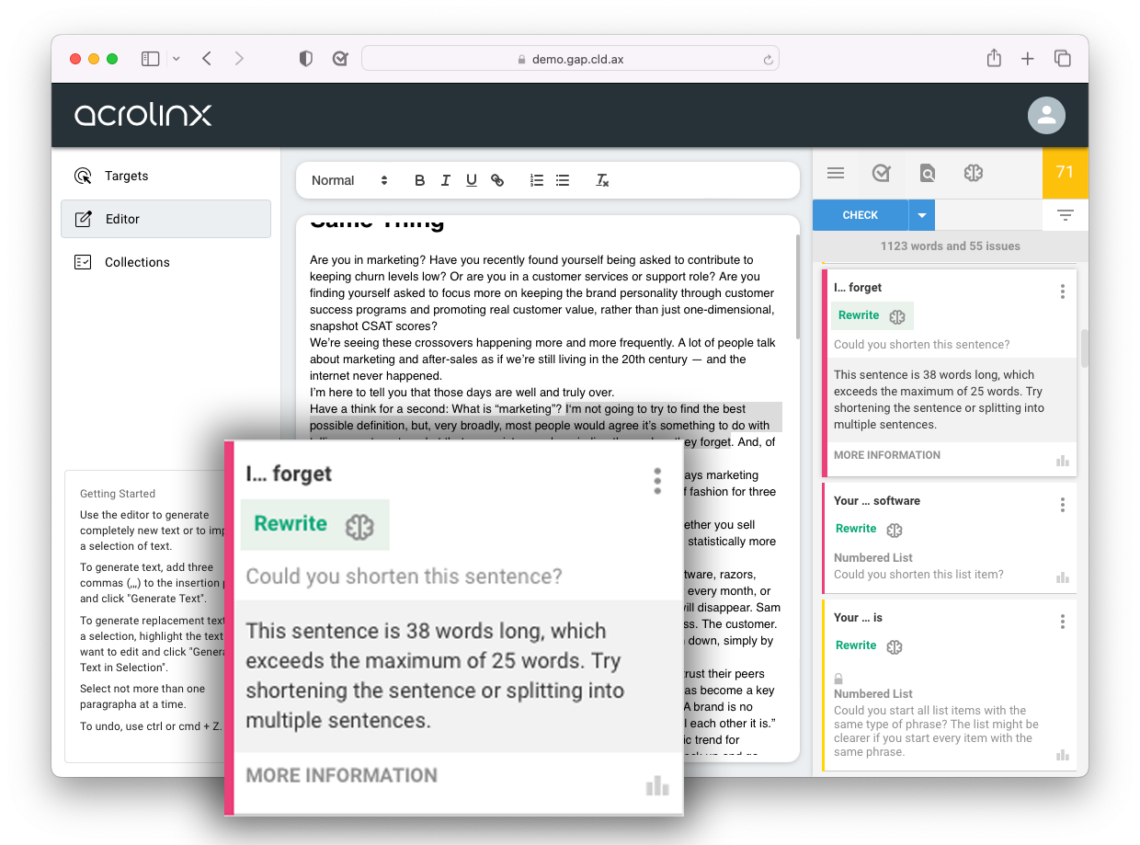
Content writers use Acrolinx to find compliance and quality issues in their content before they publish it. With dozens of integrations, Acrolinx works where your writers work. As they draft content, Acrolinx checks for issues, presents these issues, and in most cases offers a clickable replacement.
Pharmaceutical companies often automate content checking with Acrolinx. The system checks all content for quality and compliance issues, even if your writers forget to check the content while they’re creating it. Acrolinx can also implement quality gates, which check the quality of content and block it from being published if it’s below a set quality threshold. This process greatly reduces compliance risk.
Step 6 – Use content governance to avoid future compliance issues
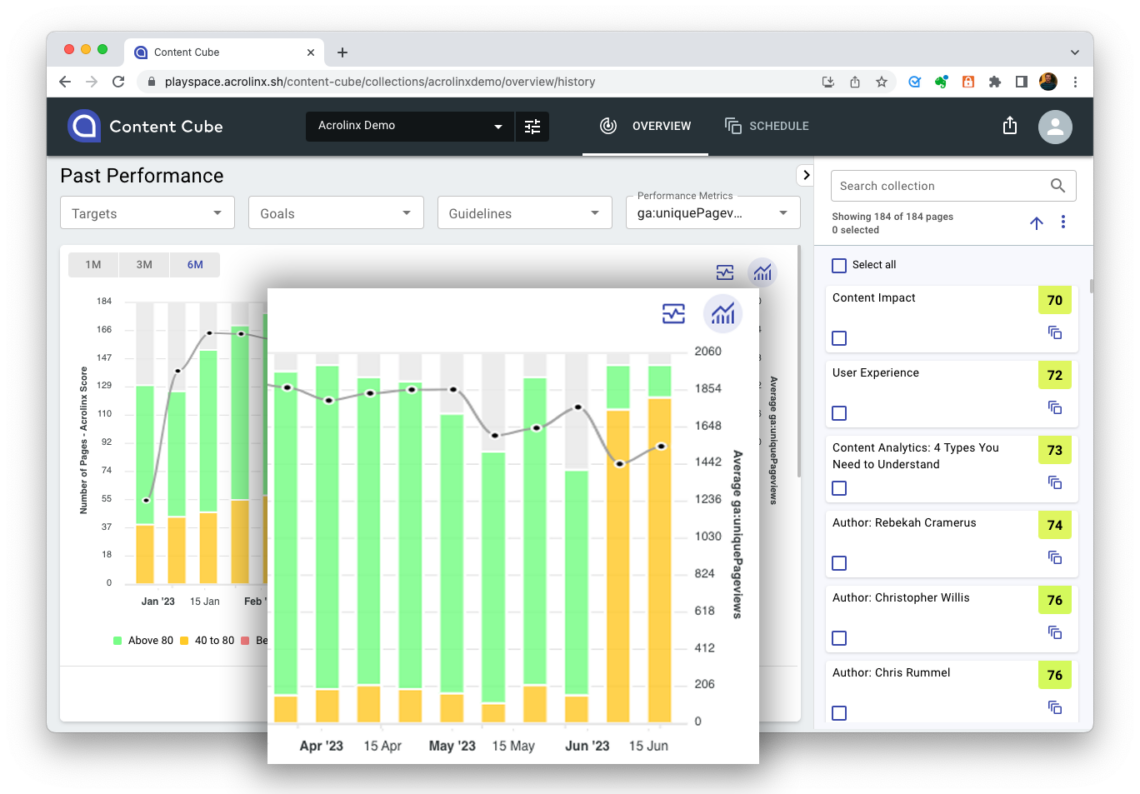
Pharmaceutical companies with mature content governance strategies use Acrolinx to continually monitor their pre- and post-published content. As content falls in quality, or compliance issues arise, Acrolinx can automate the content improvement process by identifying poor content and adding it into your content improvement workflow.
New products, research, and regulation changes require pharmaceutical companies to continuously review and update their existing content, leaving many of them exposed to compliance issues. Acrolinx reduces these risks by giving pharmaceutical companies the tools they need to find and correct issues as they arise.
Compliance is an ever-changing field for pharmaceutical companies. That’s why it’s important to stay current with changes, so you can be sure your content meets all the necessary requirements well before regulatory deadlines pass.